pH is a measure of the hydrogen ion concentration in an aqueous solution. pH is based on a negative log scale, meaning the more hydrogen ions present, the lower the pH. It’s important in many solutions to maintain a certain pH, and to do so, we use a buffer solution.
What is a buffer?
Let’s take a look at simple acidic and alkaline buffer solutions and explain how they work. A buffer is a solution that resists changes in pH when small quantities of an acid or alkali are added. Essentially, a buffer solution is typically made of a weak acid (HA) and its conjugate base. The conjugate base is often one of the weak acid’s salts, usually a sodium salt.
When many chemicals dissolve in water, they dissociate into different species and create an equilibrium.
For example, when acetic acid (a weak acid) is dissolved in water, it becomes hydrogen ions and acetate ions. All three species exist in a state of equilibrium. The acetic acid and acetate ion are the buffer system. The weak acid (acetic acid) is in equilibrium with its conjugate base (acetate ions). The pH of the buffer system remains relatively unaffected by the addition of slight amounts of acid or base.
- Acidic Buffer Solutions
Remember, acids have a pH below 7, so an acidic buffer solution simply has a pH less than 7. Acidic buffer solutions are typically made from a weak acid and one of its salts, often a sodium salt. You can change the pH of the buffer solution by changing the ratio of acid to salt, or by selecting a different acid and one of its salts.
- Alkaline Buffer Solutions
Remember, bases have a pH greater than 7, so an alkaline buffer solution has a pH greater than 7. Alkaline buffer solutions are commonly made from a weak base and one of its salts.
How Does The Buffer System Help Maintain pH?
A buffer solution contains items that will remove any hydrogen ions or hydroxide ions that you might add to it, which would result in a pH change.
In the acetic acid/acetate buffer system example above, if a small amount of acid is added to the solution, the additional hydrogen ions combine with the acetate ions to form more acetic acid. This causes the equilibrium to shift to the left, creating more acetic acid, but leaving the final hydrogen ion concentration unchanged. The result is no change in pH.
Conversely, adding a small amount of base to the solution causes the equilibrium to shift to the right. While the added base will consume the hydrogen ion present, it’s replaced by more of the acetic acid dissociating to hydrogen ions and acetate ions. Once again, the hydrogen ion concentration, or pH, remains the same.
How Are Buffer Systems Prepared?
Normally, a buffer system is prepared by dissolving a weak acid (or weak base) with a source of its conjugate base (or acid).
For example, acetic acid/acetate buffer (pH of 4.7) is created using acetic acid (weak acid) and sodium acetate (a source of the conjugate base).
What Determines the pH of a Buffer System?
The pH of a buffer solution depends on whether the weak acid or weak base is involved, the ionization constant of that weak acid or base (Ka), and the relative concentrations of the weak acid or base and its corresponding conjugate base or acid.
pH = pKa + log [conjugate base]/[weak acid]
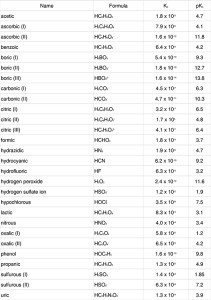
There are tables available that list buffer systems and Ka values. Here is an example from Chemistry.About.com.
Using these tables and knowing the concentration of the conjugate base and weak acid, the pH of the acidic buffer system can be calculated. The same is true for an alkaline buffer system if the conjugate acid and weak base concentrations are known.
In addition, the more concentrated the buffer pair, the greater the buffer capacity of the system, which means better resistance to pH changes.







Leave A Comment