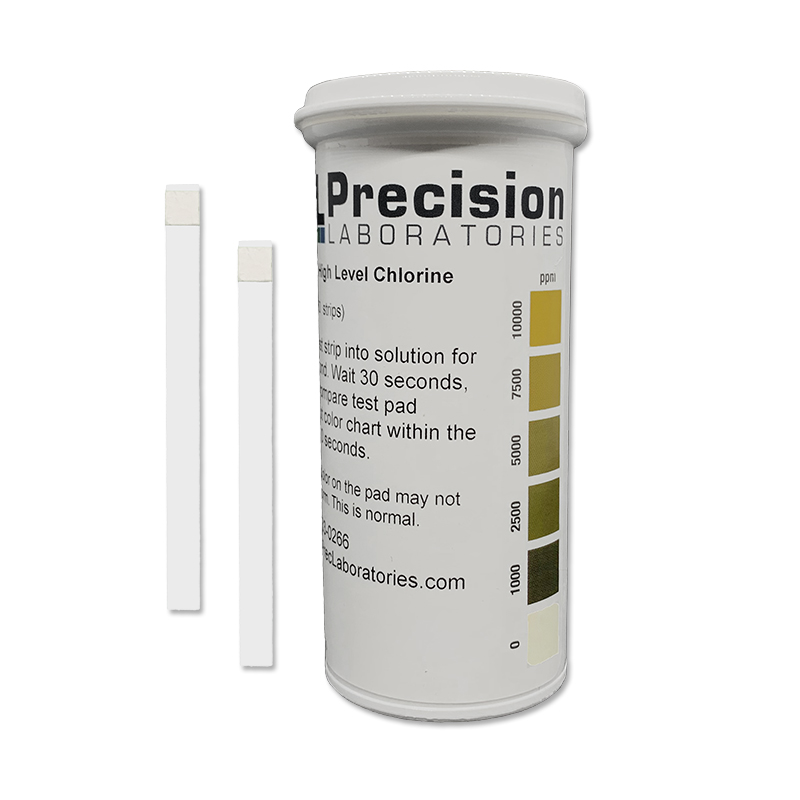
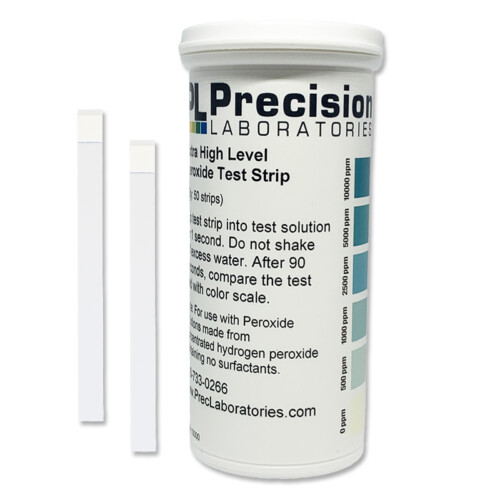
The Extra High Level Chlorine test strip tests levels of free chlorine in a solution, in steps of 0-1000-2500-5000-7500-10,000ppm, giving results in just seconds. This test strip is great for environments requiring high levels of sanitation/disinfection, such as hospitals.
Extra High Level Chlorine Test Strip, 10000ppm
Extra High Level Chlorine Test Strip, 10000ppm
- Dip a test strip into the solution for 1 second.
- Remove the strip and wait 30 seconds, then compare to the color chart within the next 10 seconds.
NOTE: These strips were designed to work with solutions prepared from bleach concentrate, and the pH of the test solution needs to be above 10 to work correctly.
NOTE: The color on the pad may not be uniform, and this is normal.
CHL10,000; CHL10,000V50; CHL10,000V100; CHL10000; CHL-10000; CHL-10000-1V-100; CHL-10000-12V-100
Product Specs
| SKU 1: | CHL-10000-1V-50 (1 vial, 50 strips/vial) |
|---|---|
| SKU 2: | CHL-10000-1V-100 (1 vial, 100 strips/vial) |
| Strip Quantity: | 100 strips, 50 strips |
| Vial Dimensions: | 1.125″ (D) x 3.375″ (H) [29mm (D) x 86mm (H)] |
| Strip Dimensions: | 2.5" (L) x .1875" (W) [64mm (L) x 5mm (W)] |
| Weight: | 0.07 lbs [33.4 g] (50-strip vial), 0.09 lbs [42.5 g] (100-strip vial) |
| Other: | Stored in a flip-top vial with built-in desiccant liner. |
| Shelf-Life: | 2 Years |
| Label: | Customizable water-resistant label |
Product Documentation
Does the colorimetric response of the Extra High Level Chlorine Test Strips (10,000ppm) have a pH dependency?
Let’s say you’re testing the free chlorine content of 1% bleach, which has a pH of 11.8. If the pH is reduced to 8, for example, will the colorimetric response of the Extra High Level Chlorine test strip for this 1% bleach change, too? Yes, it does appear that pH will influence the result.
• A 1% (10,000 ppm) hypochlorite standard was prepared from ACS hypochlorite (11.75%). The pH of the 1% solution was 11.5. This was tested with the 0-10,000 Extra High Level Chlorine test strip. The color matched the 10,000 color block very well.
• The pH of the 1% standard was lowered by adding 9 drops of concentrated HCL. The pH was 8.05. The test strip read 7,500 ppm; lower than the non-adjusted solution.
• The pH 8.05, 1% solution was diluted 1:1 with water. Theoretically, this should be 5,000 ppm. When tested with the strips, it read much closer to the 2,500 ppm color block. The pH of the diluted solution was 8.10.
• A 0.1% (1,000 ppm) standard was prepared from the original 1% standard. The pH was 10.3. The test strip results matched the 1,000 ppm color very well.
• The pH of the 1,000 ppm standard was adjusted lower by adding one drop of concentrated HCl. The pH was 8.1. The test strip read lower than 1,000 ppm.
In summary, it appears that lowering the pH to about 8 will depress the chlorine test strip result by about one color block.
What is the recommended concentration of bleach for dangerous pathogens?
Chlorine bleach at concentrations of at least 1000ppm is an effective germicide for dangerous pathogens such as MRSA (Methicillin Resistant Staphylococcus aureus), VRE (Vancomycin Resistent Enterococci) and Clostridium difficile. The High Level Chlorine ( 0-1,000ppm) test strip is suitable for use with Deardorff-Fitzsimmons ACTIVATE disinfectant (5.25% Sodium Hypochlorite solution) for in vitro control of such dangerous pathogens.
However, the removal of free chlorine by organic contamination (blood/feces) can seriously reduce the sanitizing effects of chlorine cleaning solutions. To ensure the 1000ppm minimum strength for adequate disinfection, much higher initial concentrations, up to 10,000ppm (1%) chlorine levels are recommended to counteract this and can be check with our Extra High Level Chlorine (0-10,000ppm) test strip.
How do I determine the recommended bleach concentration for a certain product?
To determine the recommended bleach concentration for your application, locate the EPA registration number on your product label. Visit the EPA’s website, and enter the EPA Registration Number, then click Search. You should see the details about the product, and beneath that, a PDF bearing the date that this product was registered by the EPA (if there is a list, the PDF at the top of the list should show the most recent approval). Click on that most recently-approved PDF. The PDF should come up on your screen. Scroll down to the section that shows the directions for using the product as a sanitizer or disinfectant. Follow the directions listed for your intended use.
How do I select the correct chlorine test strips?
Chlorine is one of the most popular chemicals for cleaning, sanitizing, and disinfecting. Two common examples will help illustrate this. Swimming pools are often treated with chlorine chemicals. In this application, the amount of chlorine needed is low, typically only 1-3ppm chlorine. On the other hand, disinfection of daycare and hospital facilities requires a much higher chlorine level, typically 600-1200ppm. In both examples, the 0-200ppm chlorine test paper is not the best choice. Precision Laboratories manufactures a whole range of chlorine test strips. Please consult our product listing for the chlorine test strip that best matches your application.
What is the ideal pH of an available chlorine solution?
All available chlorine has some biocide strength, although hypochlorous acid is a far stronger biocide than hypochlorite ion or the chloramines. In solutions at pH of 5-7, hypochlorous acid is the most prevalent species. For this reason, most sanitizing solutions will work best in the neutral to slightly acidic pH range.
What is the difference between free available, combined available, and total available chlorine?
When chlorine gas (Cl2) or bleach (sodium hypochlorite or NaOCl) is added to water the result is the formation of hypochlorous acid (HClO).
Cl2 + H2O -> HOCl + H+ + Cl-
NaOCl +H2O -> HOCl + Na+ + OH-
Depending on the pH of the solution the hypochlorous acid exists in solution as either hypochlorous acid (HClO) or hypochlorite ion (OCl-).
HOCl OCl- + H+
Free available chlorine refers to the amount of hypochlorous acid AND hypochlorite ions present.
Combined (or bound) available chlorine arises when nitrogen (usually in the form of ammonia) is present to form what are called chloramines (mono-, di-, and trichloramines are possible).
Total available chlorine is the sum of the free available chlorine and the combined available chlorine.