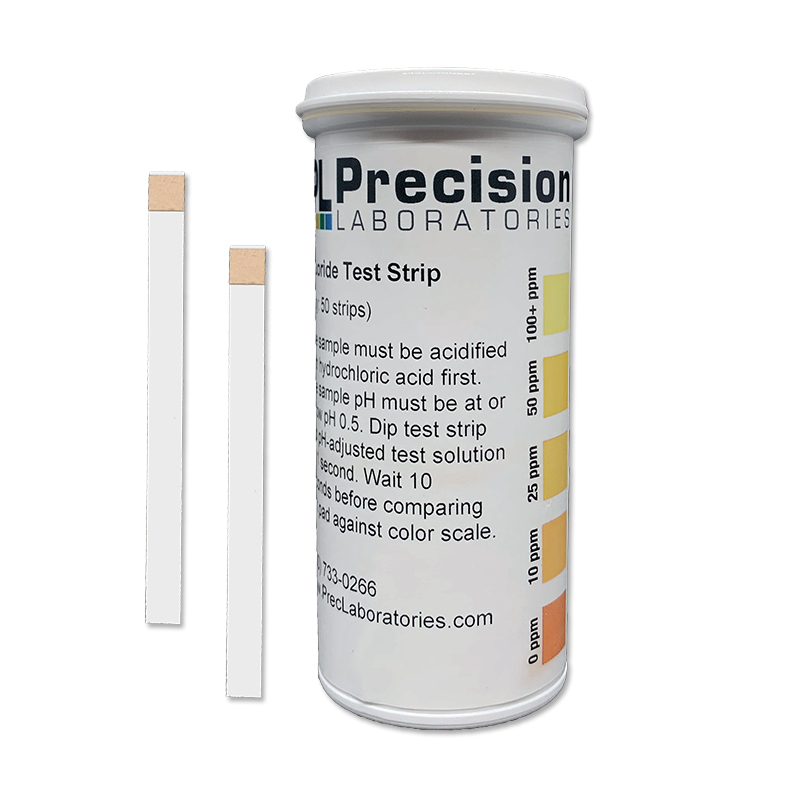
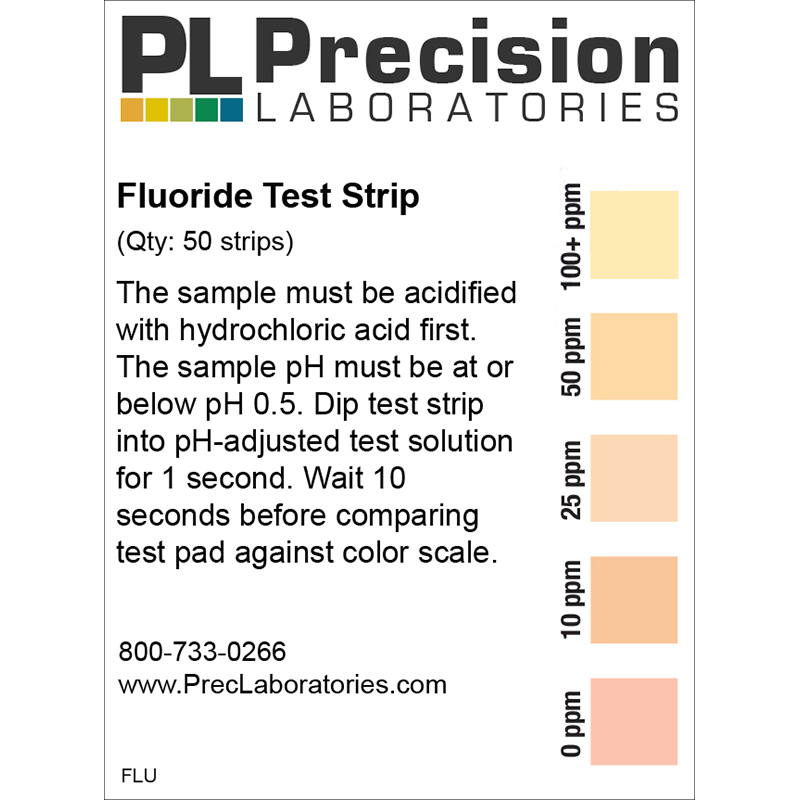
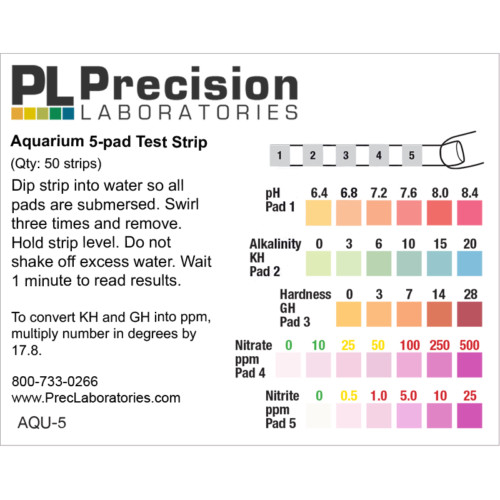
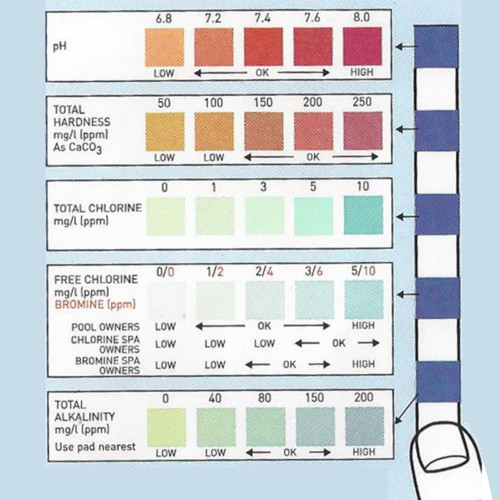
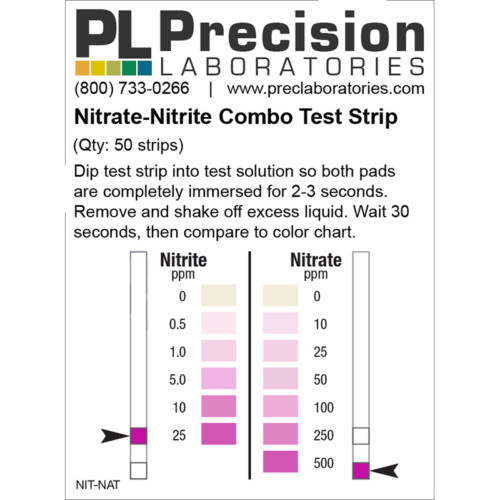
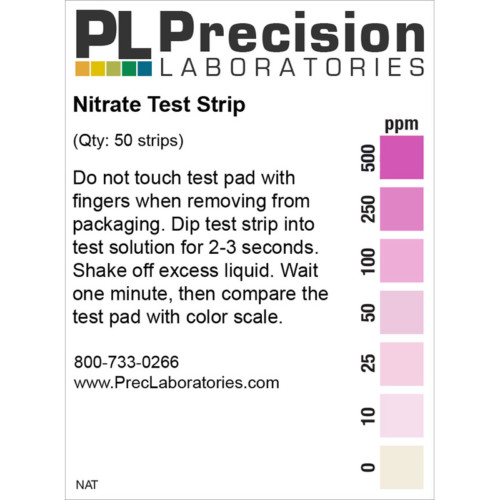
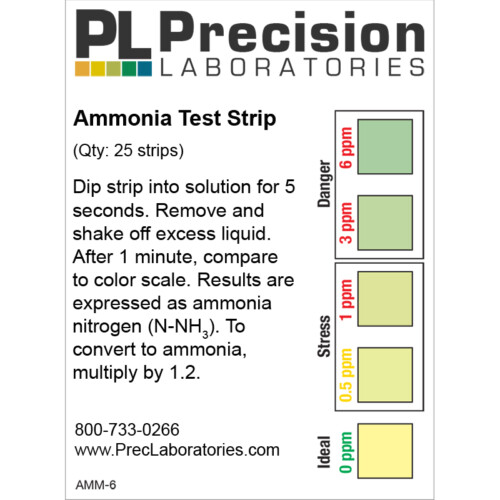
The Fluoride test strip detects Fluoride in steps of 0, 10, 25, 50, and 100ppm. This test strip is used for monitoring fluoride levels in drinking water supplied by city or townships. Too much fluoride present is harmful to humans and animals. The solution to be tested must be at a pH of 0.5 in order for this test to be accurate. This pH measure can be accomplished using concentrated Hydrochloric acid (muriatic acid) to acidify the test solution, and measured with the pH 0-1.5 test strip.
Hydrochloric acid should be available at a local hardware or pool/spa store, as well as at any chemical distributors, such as Fisher Scientific or VWR.
NOTE: The test strip result must be adjusted to compensate for the dilution used during the acidification process.