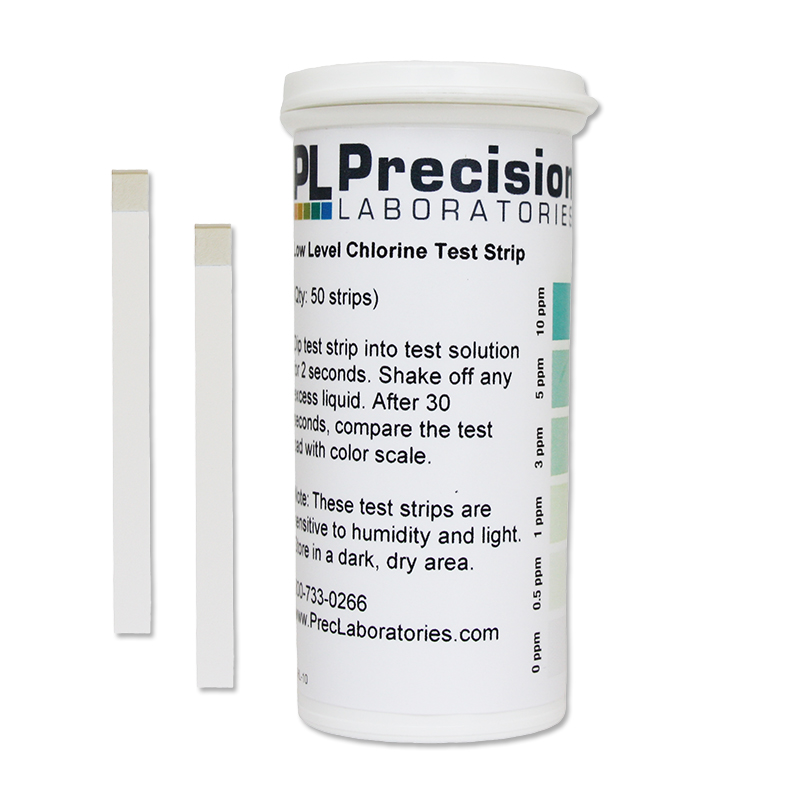
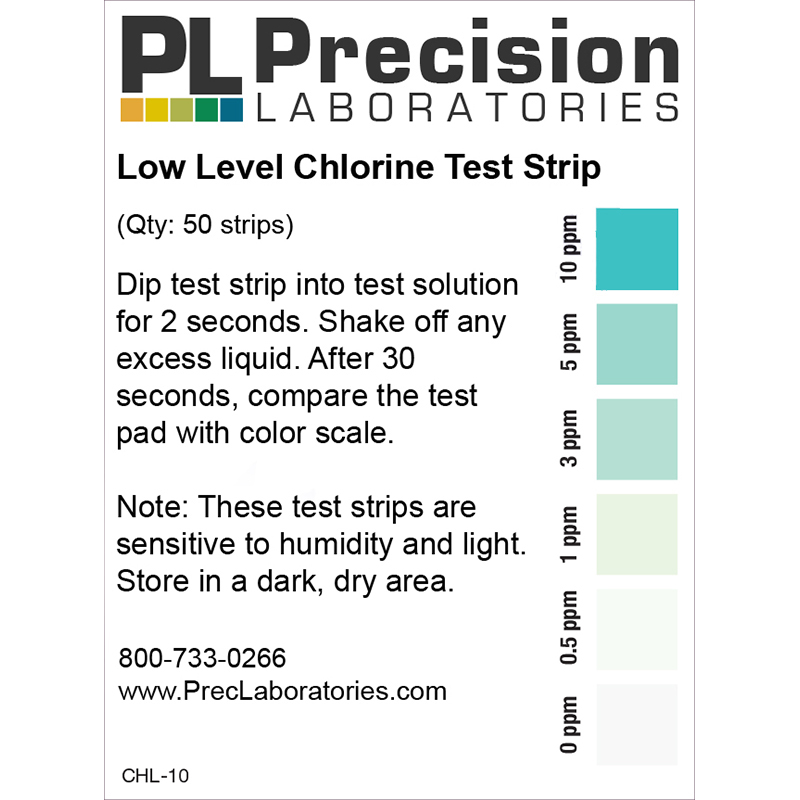
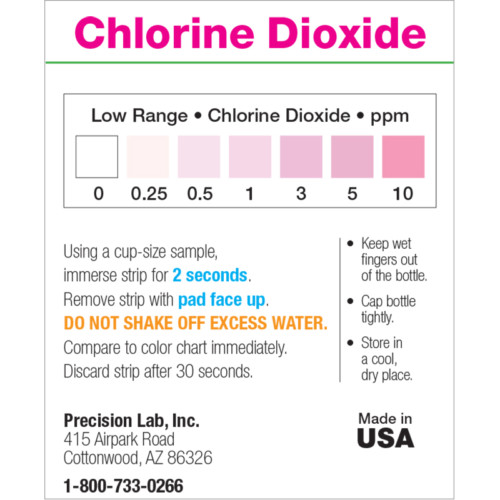
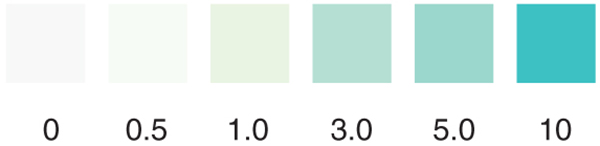
The Low Level Chlorine test strip measures residual chlorine from 0-10ppm in increments of 0-0.5-1-3-5-10 in just seconds. This test strip detects residual levels of free chlorine. It is accurate within 5-10% of reading the color scale. The strip can also be used to test for chlorine in drinking water if this is a concern, however, our Residual Chlorine test strip, which measures up to 5ppm, may be a better suited option for testing residual in drinking water.
Low Level Chlorine Test Strip, 10ppm
Low Level Chlorine Test Strip, 10ppm
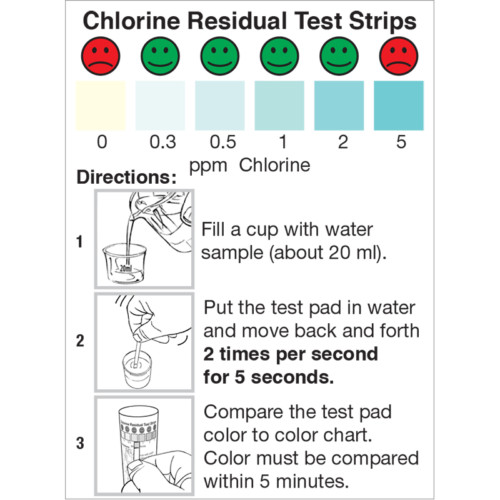
- Remove one test strip from the vial, being careful not to touch the test pad with your fingers.
- Dip the test strip into the solution being tested for 2 seconds.
- Alternatively, place a drop of deionized water on the indicator pad of the test strip, and press the pad firmly against food handling equipment, tables, or even the skin of fruit and vegetables themselves.
- Remove and shake off any excess liquid.
- Compare to the color chart after 30 seconds.
NOTE: The Low Level Chlorine test strip is sensitive to humidity and light. Store in a dark and dry area.
CHLL10; CHL-10; CHL-10-1V-50
Product Specs
| SKU: | CHL-10-1V-50 |
|---|---|
| Strip Quantity: | 50 strips |
| Vial Dimensions: | 1.125″ (D) x 3.375″ (H) [29mm (D) x 86mm (H)] |
| Strip Dimensions: | 2.5" (L) x .1875" (W) [64mm (L) x 5mm (W)] |
| Weight: | 0.07 lbs [33.4 g] (50-strip vial) |
| Other: | Stored in a flip-top vial with built-in desiccant liner. |
| Shelf-Life: | 2 Years |
| Label: | Customizable water-resistant label |
Product Documentation
What chlorine test strips can I use to test pools?
The 145 chlorine paper test strips are primarily used for monitoring restaurant and kitchen sanitizing solutions where the target ppm level is between 100-150ppm. These test papers will not work well in pools.
Swimming pool chlorine levels are usually under 10ppm free chlorine. Our CHL-10, 0-10ppm chlorine test strip will work for this application.
In addition to low levels of chlorine, pool testing is complicated by other factors (ex. water quality, biological load issues, etc). For this reason pool strips are usually designed specifically for this application. Check out our 5-pad pool test strips.
Do the Chlorine test strips only detect chlorine or do they also change color in salt concentrations (NaCl)?
Sodium chloride salt is not an oxidizer and will not react with any of our chlorine strips.
However, if you are using salt in pools as a source of chlorine, and your question is, “Can I use chlorine test strips in a salt water pool?”, the answer is yes. In this instance, you must use a chlorine test strip with the correct range, such as the Residual Chlorine 0-5ppm or Low Level Chlorine 0-10ppm test strips.
Salt is used to introduce chlorine to the water (no reaction to strips) but the salt generator or electrolysis unit takes the salt water and converts it to hypochlorous acid and sodium hypochlorite. It is the hypochlorius acid and hypochlorite ions that are commonly referred to as “available chlorine.” Hypochlorous acid is a strong oxidizer and will react with any of the chlorine strips.
What is the ideal pH of an available chlorine solution?
All available chlorine has some biocide strength, although hypochlorous acid is a far stronger biocide than hypochlorite ion or the chloramines. In solutions at pH of 5-7, hypochlorous acid is the most prevalent species. For this reason, most sanitizing solutions will work best in the neutral to slightly acidic pH range.
What is the difference between free available, combined available, and total available chlorine?
When chlorine gas (Cl2) or bleach (sodium hypochlorite or NaOCl) is added to water the result is the formation of hypochlorous acid (HClO).
Cl2 + H2O -> HOCl + H+ + Cl-
NaOCl +H2O -> HOCl + Na+ + OH-
Depending on the pH of the solution the hypochlorous acid exists in solution as either hypochlorous acid (HClO) or hypochlorite ion (OCl-).
HOCl OCl- + H+
Free available chlorine refers to the amount of hypochlorous acid AND hypochlorite ions present.
Combined (or bound) available chlorine arises when nitrogen (usually in the form of ammonia) is present to form what are called chloramines (mono-, di-, and trichloramines are possible).
Total available chlorine is the sum of the free available chlorine and the combined available chlorine.