Today’s experiment is brought to you by the US Environmental Protection Agency (EPA), a great resource for all types of information, including fun and interesting information for young scientists. In this experiment, you’re going to learn about acid rain and its effect on lakes and streams by measuring the pH of natural water located near your home or school. Rain that has become acidic from certain pollutants in the air is called acid rain. Acid rain has a pH below 7.
Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air. These substances can rise into the atmosphere where they mix and react with water, oxygen and other chemicals to form more acidic pollutants, i.e. acid rain.
So how are these compounds released into the air in the first place? More often than not, it’s due to humans. For example, sulfur dioxide is often released by power plants when they burn fossil fuels, such as coal, to produce electricity. Exhaust from cars, trucks and buses releases nitrogen oxides and sulfur dioxide into the air. All of these pollutants cause acid rain.
Although some rain is naturally acidic with a pH of about 5, humans have made it worse. Normal precipitation reacts with alkaline chemicals to neutralize natural acids. However, if the precipitation becomes too acidic, the alkaline materials may not be able to neutralize it. Over time, these neutralizing materials are washed away by acid rain, damaging crops, trees, lakes, rivers and animals.

Acid rain can cause health problems in people, but it is also damaging to nature. In this experiment, we’re going to focus on the damage to bodies of water. Without pollution or acid rain, most lakes and streams have a pH level near 6.5. However, acid rain causes them to have a much lower pH. In addition, acid rain on soil, farmland, forests and such, eventually makes its way into water. The increase in acidity can be deadly to aquatic life, including fish, amphibians, insects and plants.
 In this experiment, you’re going to measure the pH of a natural body of water near your home or school. All you need are pH test strips that cover an acidic to neutral range, such as pH 2-9 test strips, pH 0-7 test strips, or for a broader range, use pH 1-14 test strips. You will also need a cup and an adult.
In this experiment, you’re going to measure the pH of a natural body of water near your home or school. All you need are pH test strips that cover an acidic to neutral range, such as pH 2-9 test strips, pH 0-7 test strips, or for a broader range, use pH 1-14 test strips. You will also need a cup and an adult.
Go to the nearest stream, river, lake or pond with an adult. Scoop some of the surface water into the cup. Measure the pH of the water using your pH test strip. Dip one pH test strip into the water so the test pad is fully immersed for 1-2 seconds. Remove and immediately compare to the color chart. Record your results.
How acidic is the water? Based on where you live and what you have learned about acid rain, are you surprised by the result? Discuss the findings with your parents or teacher.
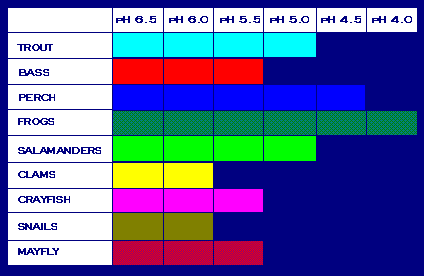
How does the measured pH compare to the pH levels that affect plants and animals in aquatic habitats? Use the chart below from the EPA to answer this question.
Going Further
To take this experiment a step further, try testing other bodies of water near your home or school to compare results. Similarly, if you live near a river, try testing the river in different locations and see how the levels compare as you move downstream. For example, is one section near farmland? How does that pH compare to upstream and downstream?
For more information on acid rain, visit the EPA’s website for additional tips and tools.







Leave A Comment