There’s something about a brightly colored pH scale and colorful pH paper that brings smiles to our faces and makes us happy. We get all starry-eyed and dreamy, and then we snap back to reality, pack it up and ship it out to you, the new proud owner of our pH test strips.
So where do all those beautiful colors come from? Why, pH indicators, of course! There are a variety of pH indicators, some coming from unexpected places like red cabbage or poinsettia plants. Then of course, there are the more common indicators, such as universal.
Below is a great infographic from Compound Chem showing the colors and ranges of pH indicators.
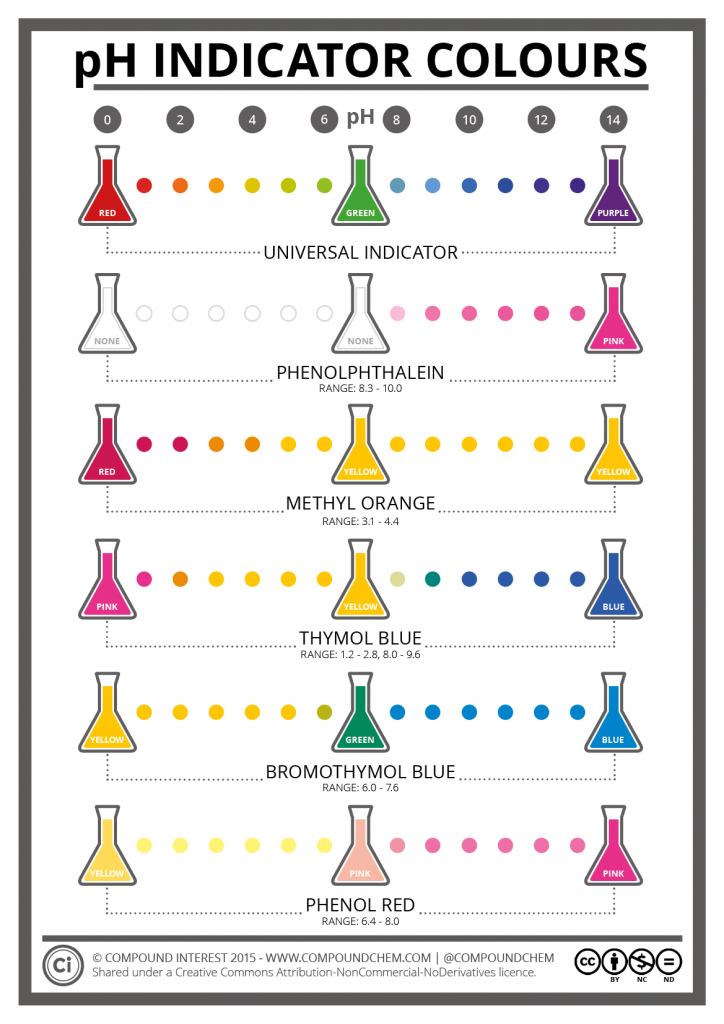
Indicators can be added to solutions to determine if the solution is alkaline or acidic. We also use indicators in our pH test strips to do the same thing. Universal indicator gives the greatest range of color changes across a broad pH range. As you might have guessed, we use universal indicator in our Universal pH Test Paper. Our Universal Test Paper detects a pH range from 2-10, going from red, orange, yellow, light green to dark green in color.
Then, there are indicators for more targeted readings that scale a narrow pH range with little color change. This is the reason for having multiple indicators, rather than relying solely on the broad range universal. It’s not as useful in situations where a more specific reading is needed. The pH scale ranges from 0-14, and the different indicators vary in what narrow range they can detect.
For example, phenolphthalein has a range of 8.3-10.0 and is useful for the titration of a strong acid with a strong alkali, or a weak acid with a strong alkali. Our Phenolphthalein Test Paper uses this indicator, which starts out on the paper as white, and turns pink in pH solutions in that range.
Other indicators include Methyl Orange (pH 3.1-4.4), Thymol Blue (pH 1.2-2.8, pH 8.0-9.6), Bromothymol blue (pH 6.0-7.6), and Phenol Red (pH 6.4-8.0). As you can see, the indicators cover varying ranges of the pH scale. Each indicator gradually changes color across the range it detects. All of our pH test papers and strips use some indicator or another.
Here’s an explanation from Compoundchem.com of what an indicator actually is:
“Indicators themselves are actually either weak acids or bases. When they’re dissolved in water, their molecules dissociate slightly and form ions. Indicators tend to be molecules containing a fair number of alternating (conjugated) carbon-carbon double bonds and single bonds, such as phenolphthalein, shown below:
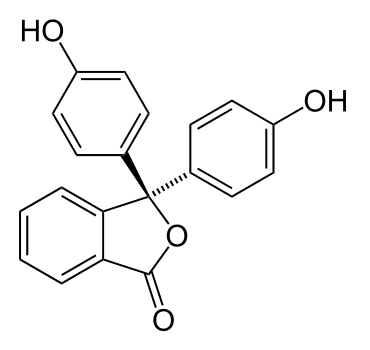
Phenolphthalein
These alternating double/single bonds can absorb wavelengths from visible light, making them appear colored. In acidic solutions, the large number of hydrogen ions already in solution means that the molecule will not dissociate much, and so the color seen will be that of the original indicator molecule. In basic solutions, however, the comparative lack of hydrogen ions in solution leads to the molecule losing a hydrogen ion; this, put simply, changes the arrangement of electrons in the molecule, causing it to absorb different wavelengths of light and appear a different color.”
Makes sense, right? And if not, just nod your head in agreement, and look at all the pretty colors on the infographic. Indicators are a pretty neat bit of science, and hopefully, you can now understand our love of pH indicators.







Leave A Comment